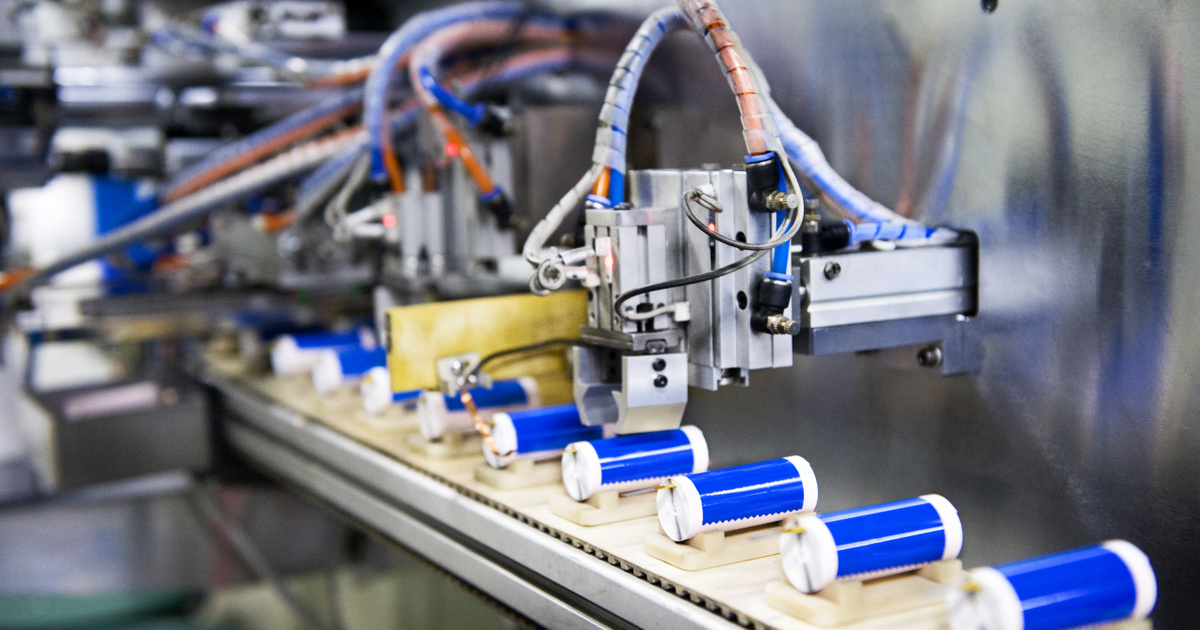
Artificial intelligence has discovered new materials that can be used to make batteries in joint research between Microsoft and the Pacific Northwest National Laboratory (PNNL). Among other things, the new materials require less lithium and allow the construction of safer batteries as a solid electrolyte. Although being integrated into devices or vehicles is still the music of the future, AI has already saved developers a lot of time.
PNNL researchers used Microsoft's Azure Quantum Cloud (AQC), a platform that combines supercomputers and artificial intelligence, to perform chemical and biochemical simulations. True to its name, AQC will eventually perform quantum computer chemistry simulations, but it's not there yet — within the framework of the current supercomputer, it has simulated the behavior of all emerging matter, including the molecular dynamics and energy levels of atoms.
The specialists wanted to find an anode material that contained less lithium. They started from 32 million possible substances, and found half a million of them stable enough for practical application. After examining the molecular properties, conductivity and production costs of the remaining materials in the competition, the list of candidates was narrowed to 800. After further study, 150 and then 23 materials remained – five of which we already knew.
Screening millions of materials would likely take a decade of work for a research group with a sufficiently stable background, but with the help of artificial intelligence, the same took only 80 hours.
PNNL employees immediately built a test battery from a material called N2116, a mixture of sodium and lithium familiar from table salt, but…
It contains 70% less lithium, also known as white gold.
Which makes today's batteries much more expensive due to the difficulty of extracting them and the high demand for them.
Experts previously knew that lithium and sodium could not be used together because their atoms have a similar charge but different sizes. But in the newly discovered material, the two elements do not hinder, but rather support each other. Moreover, the channels in the structure support the movement of ions, which is why the material also acts as a kind of solid electrolyte. The latter is a potential improvement because the liquid electrolytes found in contemporary energy storage systems tend to overheat or leak and cause fire hazards. Until now, solid electrolytes have not been able to catch on because they do not conduct energy as well as their liquid counterparts, which impairs battery performance. The problem with the material just found was that during real measurements, the current was delivered less than what AQC had predicted.
Parks are essential for spreading renewable energy and solving the climate crisis.
It will be very important to condense the next 250 years of chemistry into two decades, because we have to save the planet. As the results show, edge computing and artificial intelligence together can accelerate scientific research
– said Krista Spohr, an employee at Microsoft Research.
One PNLL official acknowledged that AQC's capabilities have led them into an entirely new, and potentially fruitful, field of chemistry, with hundreds of battery designs currently under investigation and improvement.
According to experts, since demand will increase tenfold in the coming years, and because extracting the necessary raw materials is difficult, environmentally destructive and expensive, batteries with sufficient capacity but low lithium content may represent the holy grail of future battery technology.
(BBC, Interested in trade, Extreme technology, the edge)